The Importance of Sample Pooling in COVID-19 Testing
What You Need to Know About Pooled Sample Testing
Many regions are experiencing record-breaking spikes in COVID-19 cases, with testing availability falling considerably short of demand. As vaccine distribution in the United States is just beginning, widespread testing and contact tracing remain the most effective ways to curb the recent surge in cases. The most reliable COVID-19 tests on the market use real-time reverse transcription-polymerase chain reaction (rRT-PCR) to amplify SARS-CoV-2 RNA and emit a fluorescent signal. While these tests have the benefit of being highly sensitive, they also require expensive equipment and the turn-around time is slow. Consequently, there is an urgent need to both increase sample throughput and decrease the costs associated with COVID-19 rRT-PCR tests.
One way to increase sample throughput is to use 384-well plates instead of the standard 96-well plates for rRT-PCR, which would allow four times as many RNA samples to be processed in a single run. Another option is to create multi-sample pools, which would reduce the amount of reagent used per sample and allow more samples to be tested at once.
What is Pooled Sample Testing?
Sample pooling involves testing multiple patient samples together in the same reaction 1. Prior to RNA extraction, aliquots of transport media each containing a single patient sample can be combined in one tube, or swabs from multiple patients can be added directly to the same transport media 1. Alternatively, patient samples can be processed individually to isolate RNA and then aliquots of multiple RNA samples can be pooled before rRT-PCR 2. The assay sensitivity is similar for each approach, but pooling samples before RNA extraction can save more time, money, and reagents 3.
It is necessary to select a rRT-PCR test with a very low limit of detection such as Zymo Research’s Quick SARS-CoV-2 Multiplex Kit and Quick SARS-CoV-2 rRT-PCR Kit, as sample pooling will dilute each sample and therefore decrease the amount of viral RNA from single individuals. When a multi-sample pool tests negative using rRT-PCR, it is presumed that all samples included in that pool are negative for SARS-CoV-2. If a multi-sample pool tests positive or the results are indeterminate, all samples must be individually re-tested using rRT-PCR to identify the positive case(s).
Benefits and Costs of Sample Pooling
Sample pooling can significantly increase the number of samples that can be analyzed in a given time period with the same set of resources. In a population with low to moderate virus prevalence, it is estimated that processing samples in groups of 5-10 requires 76%-93% fewer tests as opposed to individual processing 4. This ability to conserve testing reagents drastically reduces the cost associated with testing one sample. Increasing sample throughput using pooling can also expand testing availability and shorten turn-around times, allowing infected people to be informed and quarantined sooner.
One of the biggest drawbacks to sample pooling is the associated decrease in test sensitivity. Analyzing multiple samples in a single reaction effectively upscales throughput, but it also dilutes individual samples and may decrease the signal from a weak positive beyond the limit of detection. The threshold cycle (Ct) at which SARS-CoV-2 targets become detectable during quantitative PCR will increase as a positive sample is diluted with negative samples. One study recorded a linear Ct increase of 1.24 for every twofold dilution of a positive sample 2. Similarly, another study noted a 2-Ct loss in analytical sensitivity associated with using sample groups of four 5. This may cause positive SARS-CoV-2 cases with low viral load, such as newly infected patients, to be falsely classified as negative in large sample pools. To mitigate these effects, the FDA recommends that sample pooling should yield ≥85% positive agreement when compared to testing samples individually using the same rRT-PCR kit 1.
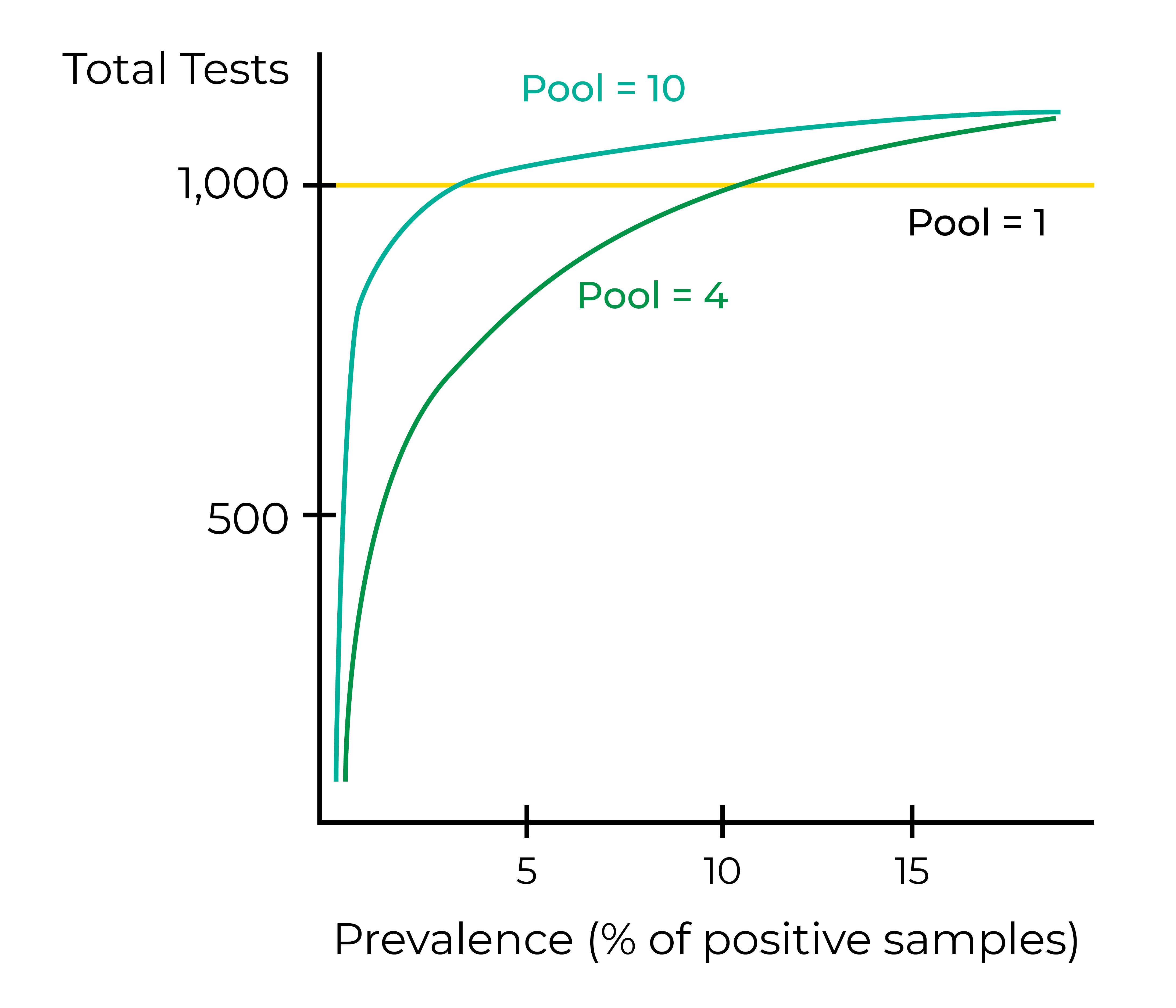
The unavoidable decrease in test sensitivity as more samples are pooled in a single reaction can be mitigated by increasing the frequency of testing. Because sample pooling conserves testing reagents and dramatically reduces the cost per sample, there is a potential to expand COVID-19 screening with rRT-PCR tests. A mild decrease in test sensitivity may not be problematic if testing is performed at a high frequency, as the probability of receiving two false-negative results with rRT-PCR is very low 3. While a decrease in test sensitivity may be acceptable for screening services in asymptomatic populations, the accuracy of results must be prioritized when providing a diagnosis to high-risk or symptomatic patients.
How Many Samples can be Pooled?
The optimal number of samples to include in a pooled reaction is inversely related to the prevalence of COVID-19 in the population. No sample pooling or fewer samples should be used per reaction when disease prevalence is high to minimize the delay in reporting results and the cost associated with re-testing all samples in pools that initially tested positive. In a population with COVID-19 prevalence <0.8%, pooling samples in groups of 20 results in the fewest tests required3. When prevalence exceeds 3%, samples should be pooled in groups of no more than five to minimize costs and tests required 3. Using a model in which the disease prevalence is unknown, another study concluded the optimal sample number per pool to be four 6.
Even in populations with very low disease prevalence, the number of samples per pool should be limited to preserve the sensitivity of the assay. One study reported that SARS-CoV-2 positive samples with low viral load (>33 Ct when tested individually) were no longer detectable when pooled with more than four negative samples 7. Another study estimated that test sensitivity was reduced by 7.41%, 11.11%, and 14.81% when sample pool sizes of 5, 10, and 20 were used, respectively 3. In general, the more samples pooled in a single reaction, the higher the false-negative rate becomes.
When Should Sample Pooling be Used?
Sample pooling is most practical in communities with low positivity rates, as the cost savings in terms of time, money, and reagents increase as the SARS-CoV-2 incidence decreases 3. In regions with high disease prevalence (for example, >10%), there would be very little to no benefit of pooled testing due to the need to individually re-test all samples in pools that test positive 5, with consequent increased testing costs and delayed results. Sample pooling would also be useful for screening asymptomatic individuals with no known exposure to the virus, such as people who need to be cleared to return to work, school, or sports 1.
Pooled sample testing for COVID-19 has the potential to increase throughput and accommodate rising demand in low-risk populations. The prevalence of COVID-19 in the population, the projected frequency of testing, and the effect of pooling on assay sensitivity must be weighed when considering whether sample pooling is beneficial and to what degree.
Learn more about the Quick SARS-CoV-2 Multiplex Kit, a solution for highly sensitive and high-throughput COVID-19 testing:
Learn moreReferences:
1. Center for Devices and Radiological Health. Pooled Sample Testing and Screening Testing for COVID-19. U.S. Food and Drug Administration. Published 2020 Aug 24. https://www.fda.gov/medical-devices/coronavirus-covid-19-and-medical-devices/pooled-sample-testing-and-screening-testing-covid-19
2. Yelin I, Aharony N, Tamar ES, et al. Evaluation of COVID-19 RT-qPCR test in multi-sample pools. Clinical Infectious Diseases 2020; 71(16): 2073-2078. Published 2020 May 2. DOI: https://doi.org/10.1093/cid/ciaa531.
3. Watkins AE, Fenichel EP, Weinberger DM, et al. Pooling saliva to increase SARS-CoV-2 testing capacity. medRxiv. Published 2020 Sep 3. DOI: https://doi.org/10.1101/2020.09.02.20183830
4. Garg J, Singh V, Pandey P, et al. Evaluation of sample pooling for diagnosis of COVID‐19 by real time‐PCR: A resource‐saving combat strategy. Wiley Online Library. Published 2020 Sep 29. DOI: https://doi.org/10.1002/jmv.26475
5. Perchetti GA, Sullivan K-W, Pepper G, et al. Pooling of SARS-CoV-2 samples to increase molecular testing throughput. Journal of Clinical Virology. Published 2020 Aug 2. DOI: https://doi.org/10.1016/j.jcv.2020.104570
6. Pikovski A, Bentele K. Pooling of coronavirus tests under unknown prevalence. Epidemiol Infect. 2020; 148:e183. Published 2020 Aug 6. DOI: 10.1017/S0950268820001752
7. Salimnia H, Mitchell R, Gundel A, et al. Pooling samples: a testing option for SARS-CoV-2 during a supply shortage [published online ahead of print, 2020 Sep 11]. Diagn Microbiol Infect Dis. 2020; 99(1):115205. DOI: 10.1016/j.diagmicrobio.2020.115205.


