The First 510(k)-Cleared Transport Medium for COVID-19 Testing



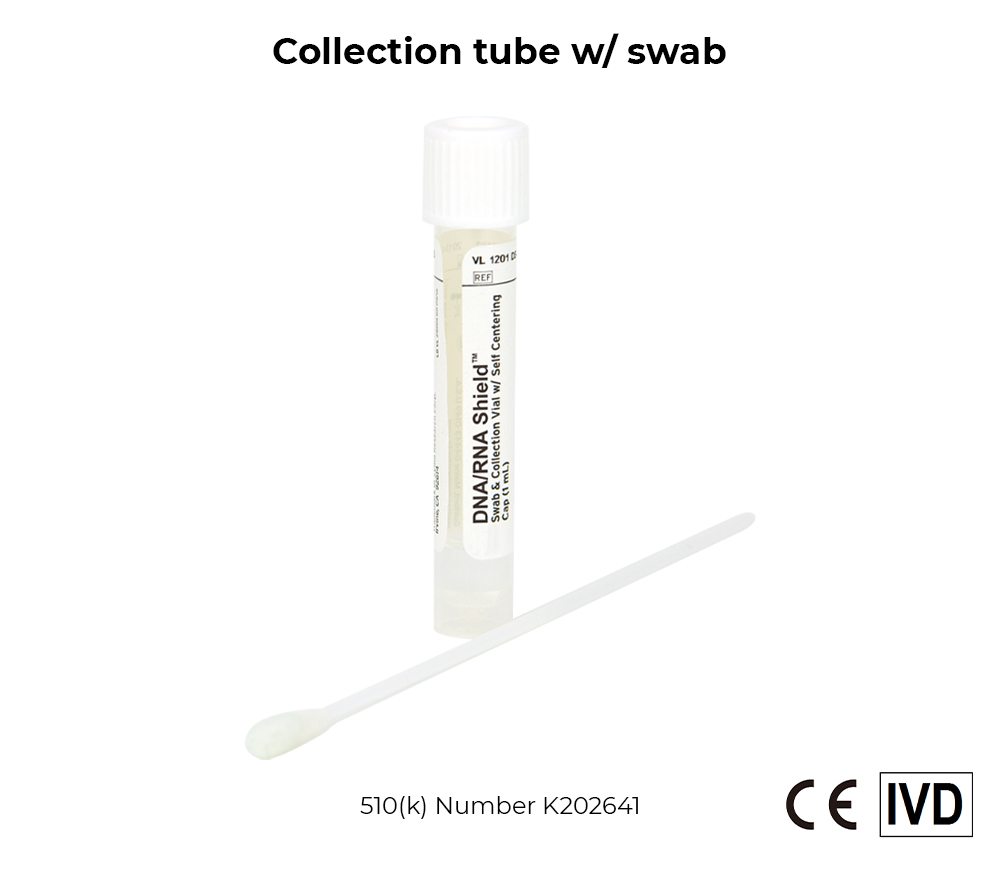
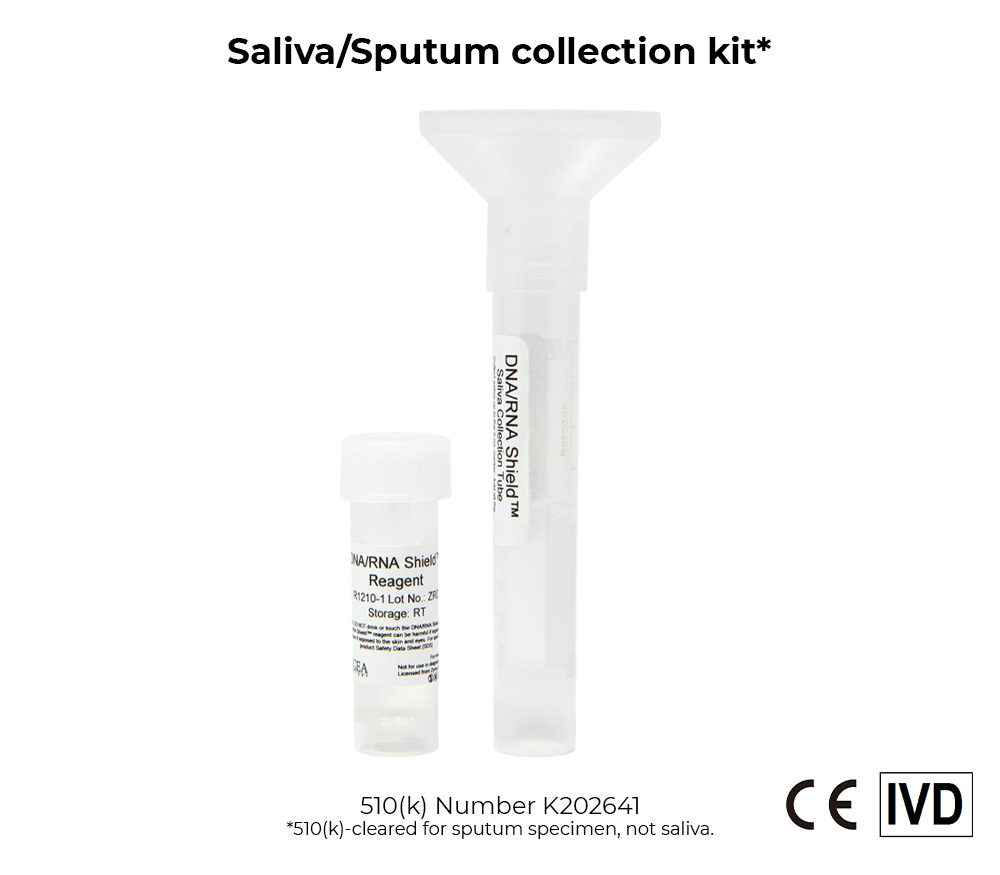
DNA/RNA Shield Collection Devices
FDA-Cleared Transport Medium Intended For Use In COVID-19 Diagnostics
Intended Use
The DNA/RNA Shield collection tube is intended for the stabilization and inactivation of upper and lower respiratory human specimens suspected of containing SARS-CoV-2. These devices can be used for collection transport and storage of specimens at ambient temperatures (20-25°C). Specimens collected and stored in a DNA/RNA Shield collection tube are suitable for use with legally marketed molecular diagnostic services.
Device Description
The DNA/RNA Shield collection tube and reagent consist of a tube pre-filled with DNA/RNA Shield transport medium. DNA/RNA Shield is a transport medium that ensures stability of SARS-CoV-2 RNA during sample transport/storage at ambient temperatures and is intended to inactivate SARS-CoV-2, effectively lyses cells from collected upper and lower respiratory biological specimens. The DNA/RNA Shield transport medium may be used in conjunction with a swab, sputum collection kit, or as a tube alone.
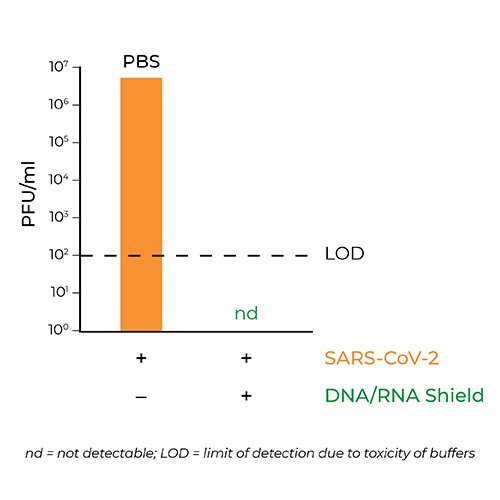
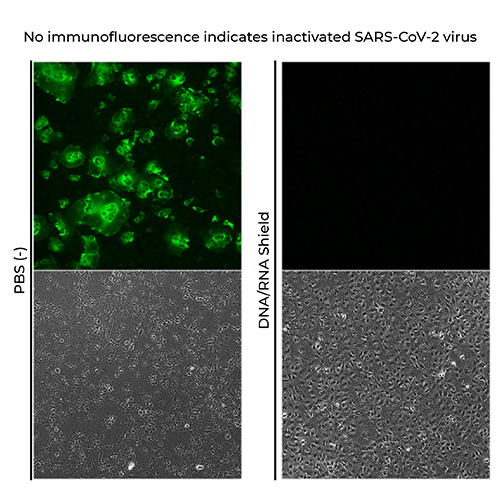
Inactivation of SARS-CoV-2
Martin Schwemmle, PhD, Institute of Virology - Medical Center, Freiburg University, Germany
Takashi Irie, PhD, Department of Virology, Institute of Biomedical & Health Sciences, Hiroshima University
The results showed that SARS-CoV-2 virus mixed with DNA/RNA Shield was completely inactivated.
Unparalleled Stability
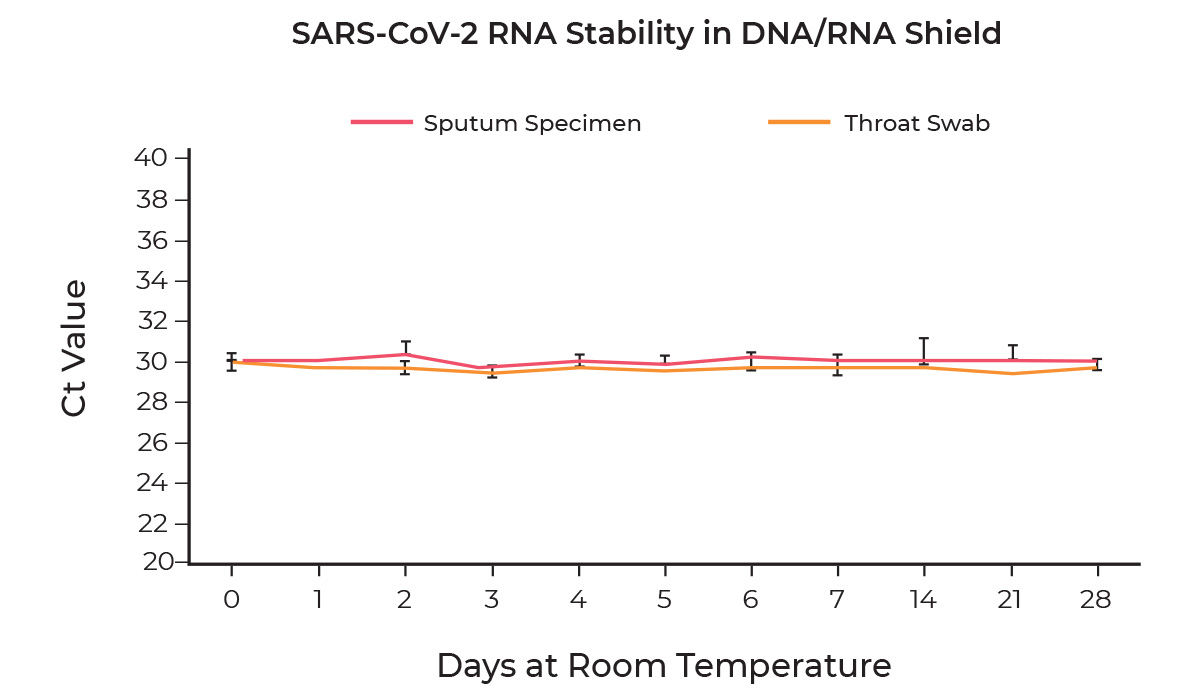
The stability of SARS-CoV-2 RNA in DNA/RNA Shield was determined by spiking 1,250 GEC/ml of viral RNA into DNA/RNA Shield alone, sputum specimens collected using the DNA/RNA Shield Saliva Collection Kit, or throat swab specimens collected using the DNA/RNA Shield Swab Collection Kit and stored at room temperature for 28 days. Samples were extracted using the Quick-DNA/RNA Viral MagBead kit performed on the KingFisher™ Flex Purification System at day 0, 1, 2, 3, 4, 5, 6, 7, 14, 21, and 28 and the stability of the viral RNA was measured using the Quick SARS-CoV-2 rRT-PCR Kit. Duplicate samples were independently processed at each time point. Based on evaluation of Ct values, SARS-CoV-2 viral RNA remains stable in all samples over the 28-day period.
What People Are Saying About DNA/RNA Shield

“We rely on Zymo Research sample collection tubes for acute SARS-CoV-2 diagnostics. We value the inactivating capabilities of the CE IVD-certified DNA/RNA Shield solution, which allows us safer sample handling and results in higher throughput in our lab.”

“A critical part of Curative's success developing and scaling our easy-to-use oral fluid COVID-19 test for widespread use across the country has been our ability to identify alternative resources like Zymo Research's reagents. We're so pleased to have a strong partner in Zymo as we continue to deliver testing resources to communities across the country, including Los Angeles, Chicago, Delaware, Texas, and more.”