Successfully Added to Cart
Customers also bought...
-
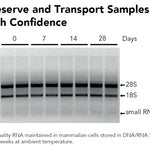 DNA/RNA Shield (50 ml)Cat#: R1100-50DNA/RNA Shield reagent is a DNA and RNA stabilization solution for nucleic acids in any biological sample. This DNA and RNA stabilization solution preserves the...
DNA/RNA Shield (50 ml)Cat#: R1100-50DNA/RNA Shield reagent is a DNA and RNA stabilization solution for nucleic acids in any biological sample. This DNA and RNA stabilization solution preserves the... -
 DNA/RNA Shield SafeCollect Swab Collection Kit (1 ml fill) (1 collection kit)Cat#: R1160The DNA/RNA Shield SafeCollect Swab Collection Kit is a user-friendly collection kit for stabilizing the nucleic acid content of samples collected with a swab. DNA/RNA...
DNA/RNA Shield SafeCollect Swab Collection Kit (1 ml fill) (1 collection kit)Cat#: R1160The DNA/RNA Shield SafeCollect Swab Collection Kit is a user-friendly collection kit for stabilizing the nucleic acid content of samples collected with a swab. DNA/RNA...
Highlights
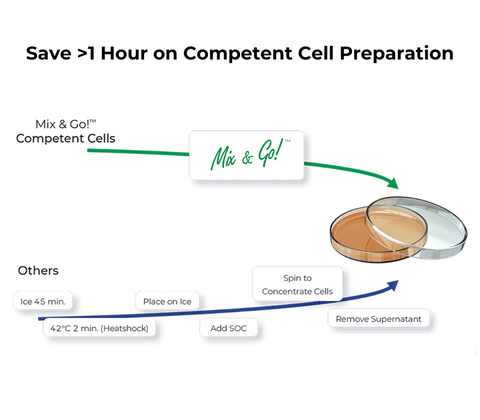
- Simple 20 Second Transformation: No heat shock! Just add DNA and spread on plate.
- High Transformation Efficiencies: Achieve 108 - 109 per µg of plasmid DNA.
- Versatile: Excellent for general cloning and plasmid isolation.
Original Manufacturer
Satisfaction 100% guaranteed, read Our Promise
Innovated in California, Made in the USA
Highlights
- Simple 20 Second Transformation: No heat shock! Just add DNA and spread on plate.
- High Transformation Efficiencies: Achieve 108 - 109 per µg of plasmid DNA.
- Versatile: Excellent for general cloning and plasmid isolation.
Original Manufacturer
Satisfaction 100% guaranteed, read Our Promise
Innovated in California, Made in the USA
Description
Performance
Technical Specifications
| Additional Info | Streptomycin Resistant (StrR) and can not be used for Blue-White Selection. |
|---|---|
| Genotype | F- Δ(gpt-proA)62 leuB6 glnV44 (supE44) ara-14 galK2 lacY1 Δ(mcrC-mrr) xyl-5 mtl-1 recA13 thi-1 rpsL20 (SmR) |
| Processing Time | 20 Seconds |
| Product Storage | -70°C to -80°C |
| Transformation Efficiency | 108 - 109 transformants per µg of plasmid DNA |
Resources
Documents
FAQ
All our competent cells are classified into Biosafety level 1 and are not genetic modified organisms. Only when transformed with a plasmid they become GMOs.
Most cloning strains will be dam+/dcm+ unless specifically noted in the genotype.
Yes
DH5α is equivalent to Zymo 5α. DH10B, Top10, and One Shot Top10 are equivalent to Zymo 10B. For XL-21 Blue, JM109 is the closest match and for Stbl3, HB101 is the closest match.
– Prepare fresh agar plates – Use more antibiotics in plates – Incubate plates for a shorter time after plating cells
We do not recommend diluting the competent cells. We recommend using less DNA to transform cells, or aliquot cells in smaller volumes before transformation. If absolutely necessary, cold 1X Competent Buffer (Mix & Go Transformation Kit, T3001 & T3002) should be used in the dilution.
No outgrowth is necessary when using Ampicillin or Carbenicillin for selection. However, an outgrowth step is required when using Chloramphenicol, Kanamycin, and Tetracycline because of the mode of action of the antibiotic itself. We recommend the following procedure for the outgrowth step: 1. Incubate cells on ice for 5-10 min after addition of plasmid. 2. Add 4 volumes of SOC media. 3. Incubate at 37°C for 60 min with gentle shaking at 200-300 rpm. 4. Spread on a pre-warmed culture plate containing the appropriate antibiotic.
For Zymo 5α and Zymo 10B up to 20kb. However, transformation efficiency decreases proportionally from 10-20kb. Above 20kb, cells are difficult to transform. JM109, HB101, XJa, XJa (DE3), XJb, XJb (DE3) and TG1 can handle constructs up to 10kb.
There really is no maximum or minimum recommended DNA concentration, but we use 10 pg for quality control. However, the volume of DNA added should not exceed 5% of the cells total volume; the efficiency can decrease several fold as the volume of DNA used increases. If the DNA sample is too diluted, use our DNA Clean & Concentrator.
1. Thaw cells on ice, not room temperature. 2. Incubate cells and DNA mixture on ice, not at room temperature. However, do not incubate longer then 1 hour. 3. Ensure cells are still frozen when received. 4. Pre-warm the culture plates at 37°C for at least 30 minutes. 5. Prepare fresh LB agar plates containing the appropriate antibiotic. 6. Prepare a new DNA sample. 7. Store the cells at -80°C (not 4°C or -20°C). If the freezer breaks, the cells should be OK as long as the temp does not go higher than -50°C. 8. Avoid freeze/thaw cycles.
Heat shock is not necessary, however sometimes it can be beneficiary when preparing libraries or transforming XJb Autolysis E. coli strains. We recommend the following protocol for Heat Shock with Outgrowth: 1. Incubate cells on ice for 5-10 min after addition of plasmid. 2. Incubate cells at 42°C for 45 seconds. 3. Add 450 ml of SOC to the cells. 4. Incubate at 37°C for 60 min with gentle shaking at 200-300 rpm. 5. Spread on a pre-warmed culture plate containing the appropriate antibiotic.
Product Video
Citations
Need help? Contact Us