Over the past 50 years, plasmid DNA has proven to be an indispensable biological tool. With a wide variety of applications, plasmid DNA is utilized in biotech, academia, and clinical laboratories. Read on to learn about plasmid structure, how to select an appropriate ORI, how to control plasmid copy number, and how plasmid DNA is routinely used in the lab.
Plasmid DNA Fundamentals
Plasmid DNA is a small, circular ring of extrachromosomal DNA that can replicate independently of genomic DNA, enabling one bacterial cell to have multiple plasmid copies. Plasmid DNA often contains genes that confer functional benefits to the host, such as antibiotic resistance or virulence. Some essential elements of plasmid DNA for use in research include:

- Origin of Replication (ORI): The DNA sequence where replication of a plasmid is initiated by bacterial replication proteins.
- Antibiotic Resistance Gene: The gene which confers antibiotic resistance to the bacteria carrying the plasmid. Antibiotics are added to select for bacteria that have the plasmid.
- Selectable Marker: Selectable markers are used to identify host cells that have taken up the plasmid. Unlike the antibiotic resistance gene, the selectable marker is typically expressed in the organism of interest and used to confirm uptake of the plasmid DNA.
- Promoter Region: A sequence of DNA that initiates the transcription of the inserted gene. This plays a key role in regulating the expression of the gene of interest in specific cell types.
- Insert: The gene of interest that is cloned into the plasmid.
- Multiple Cloning Site (MCS): A region of the plasmid that contains various restriction sites. This allows for the insert's incorporation into the plasmid via molecular cloning.
ORIs
There are several ORIs to choose from, each with varying characteristics. A few important things to consider when choosing an ORI are desired plasmid copy number, host organism, plasmid compatibility and intended application. ORIs can be classified as either relaxed or stringent. Relaxed ORIs do not need host replication initiation proteins to start replication whereas stringent ORIs rely on host initiation proteins to start replication. As a result, relaxed ORIs typically have higher copy numbers. Below is a table summarizing some common ORIs and their compatibility.
| ORI | Incompatibility Group | Control |
|---|---|---|
| ColE1 | A | Relaxed |
| PMB1 | A | Relaxed |
| PSC101 | C | Stringent |
| R6K | C | Stringent |
| 15A | B | Relaxed |
Tips for Success: Plasmids with the same ORI should NOT be co-transformed! Two plasmids with the same ORI will be in competition for the same replication machinery, causing unpredictable results.
Plasmid Copy Number
Plasmid copy number is the number of plasmid DNA copies in a single host cell and can range anywhere from a dozen to several hundred. As a result, it is good practice to know whether your plasmid vector is high, medium, or low copy to predict an accurate expected yield. Below is a table summarizing the origin of replication and copy number of several common plasmids.
| Plasmid Name | ORI | Copy Number | Classification |
|---|---|---|---|
| pUC | pMBI Derivative | 500-700 | High Copy |
| pGEM | pUC | 300-500 | High Copy |
| pBR322 | pMBI | 15-20 | Low Copy |
| pET | pBR322 | 15-20 | Low Copy |
| pColE1 | ColE1 | 15-20 | Low Copy |
| pBluescript | ColE1 Derivative | 300-500 | High Copy |
| pCDNA3.1TM | pUC | 300-500 | High Copy |
| pLenti6.2/V5-DEST | pUC | 300-500 | High Copy |
In addition to the ORI, the factors listed below can also influence plasmid copy number and yield:
- Larger plasmids will have a lower copy number as the increased size leads to a greater metabolic burden on the cell.
- Inserting an extra-large DNA fragment into a high copy plasmid may cause it to behave more like a low copy plasmid./li>
- Some plasmids, like pUC plasmids, can achieve exceedingly high copy numbers by including a mutation that prevents the inhibition of plasmid replication.
- Plasmids that contain an insert or gene that produces a toxic product tend to have a low copy number.
- Plasmids are unstable in certain E. coli strains and suboptimal culturing conditions can impede cell growth. Read our "How To Increase Plasmid Yield" resource for a more in-depth guide on how to troubleshoot low plasmid yield.
Preparing Plasmid for Use in the Lab
Plasmid DNA has a wide range of uses in the life sciences, from gene therapy to protein expression. However, nearly all plasmid applications require creating recombinant plasmid DNA and recovering high-purity plasmid. As a result, several of the steps for preparing plasmid are ubiquitous. Below is a typical workflow for preparing recombinant plasmid DNA:
-
Cloning – The gene of interest is integrated into a plasmid vector, resulting in functional recombinant plasmid DNA. Traditionally restriction enzymes are used to cut the gene of interest and plasmid vector at specific sites (see diagram below), but there are many other modern cloning methods including gateway cloning, Gibson assembly, and TOPO Cloning.
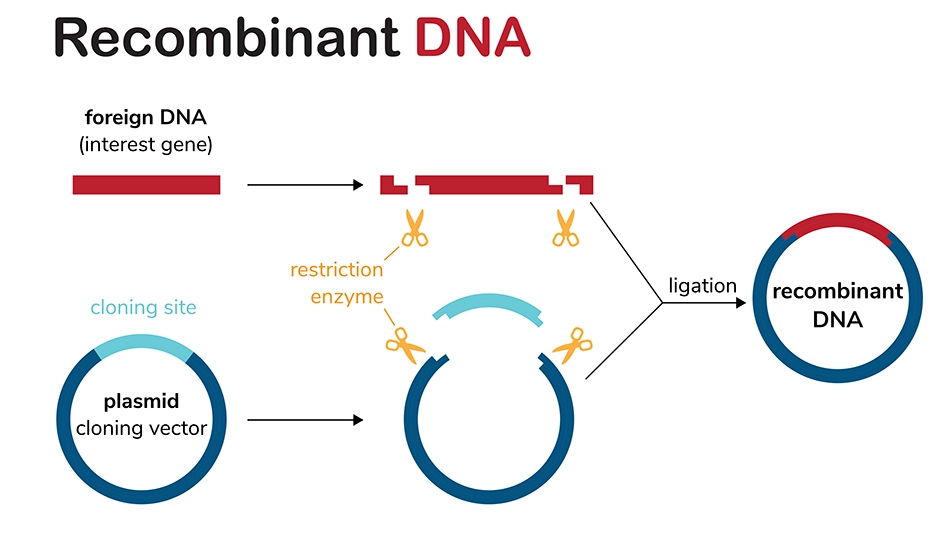
-
Transformation – Next, the recombinant plasmid is transformed into competent bacteria (bacteria that can take up foreign DNA from the environment). Typically, E. coli is used because it is a well-studied microorganism and easy to grow. However, E. coli is NOT naturally competent and must be made competent by physical or chemical means to take up the recombinant plasmid. Zymo’s Mix & Go! E. coli Competent Cells are designed for simple, high-efficiency transformations without the need for heat shock or lengthy incubation steps. To select for bacteria that have successfully taken up the plasmid, transformed bacteria is grown in antibiotic-containing media. Bacteria containing the antibiotic-resistance encoding plasmid will survive, all others will be susceptible to the antibiotics and die.
-
Plasmid Propagation – To grow more bacteria that have successfully taken up the plasmid DNA, transformed bacteria is grown in antibiotic-containing liquid media. In allowing the transformed bacterial cells to grow and divide, this propagation step increases the number of plasmid copies. Read our 7 Expert Tips for Bacterial Culture for more information on how much antibiotic to add to your growth media and other helpful tips for optimizing plasmid propagation.
-
Plasmid Isolation & PurificationOnce the culture is grown up to an appropriate density, recombinant plasmid DNA is purified from the bacterial culture for use in downstream applications. The purified plasmid can be transformed into bacteria or transfected into eukaryotic cells for study in the host cell. Ensure that your isolated plasmid is suitable for all downstream applications with Zymo Research’s ZymoPURE Plasmid Purification Kits,, the fastest & most efficient method to purify transfection-grade plasmid DNA from E. coli. ZymoPURETM is highly cited for plasmid applications such as:
- Gene Therapy
- Vaccine Development
- Recombinant Antibody Therapy
- Protein Expression
- Gene Editing
- Viral Vector Production
Interested in more plasmid-related content? Check out Zymo’s dedicated collection of plasmid resources for information on everything plasmid, from pieces on cutting edge research using plasmid DNA to helpful guides for plasmid purification success.
Scientists at Zymo Research know the importance of recovering quality plasmid and have developed plasmid purification technologies with your success in mind. Ensure you recover the most concentrated, highest-purity plasmid DNA possible with our ZymoPURE™ Plasmid Purification product line.
