Successfully Added to Cart
Customers also bought...
-
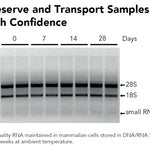 DNA/RNA Shield (50 ml)Cat#: R1100-50DNA/RNA Shield reagent is a DNA and RNA stabilization solution for nucleic acids in any biological sample. This DNA and RNA stabilization solution preserves the...
DNA/RNA Shield (50 ml)Cat#: R1100-50DNA/RNA Shield reagent is a DNA and RNA stabilization solution for nucleic acids in any biological sample. This DNA and RNA stabilization solution preserves the... -
 DNA/RNA Shield SafeCollect Swab Collection Kit (1 ml fill) (1 collection kit)Cat#: R1160The DNA/RNA Shield SafeCollect Swab Collection Kit is a user-friendly collection kit for stabilizing the nucleic acid content of samples collected with a swab. DNA/RNA...
DNA/RNA Shield SafeCollect Swab Collection Kit (1 ml fill) (1 collection kit)Cat#: R1160The DNA/RNA Shield SafeCollect Swab Collection Kit is a user-friendly collection kit for stabilizing the nucleic acid content of samples collected with a swab. DNA/RNA...
Highlights
- Approved for emergency use authorization (EUA) by the U.S. Food and Drug Administration and CE-IVD certified.
- High Sensitivity: Ranked among the top three most sensitive EUA tests by the FDA, with a Limit of Detection as low as 250 GEC/ml (15 GEC/reaction).
- Specific: Detection of SARS-CoV-2 and Emerging Strains (including Omicron)
- Rapid & Easy Setup: Ready-to-use Master Mix, just add sample.
- Compatible with Automated and High-Throughput workflows.
Original Manufacturer
Satisfaction 100% guaranteed, read Our Promise
Innovated in California, Made in the USA
Highlights
- Approved for emergency use authorization (EUA) by the U.S. Food and Drug Administration and CE-IVD certified.
- High Sensitivity: Ranked among the top three most sensitive EUA tests by the FDA, with a Limit of Detection as low as 250 GEC/ml (15 GEC/reaction).
- Specific: Detection of SARS-CoV-2 and Emerging Strains (including Omicron)
- Rapid & Easy Setup: Ready-to-use Master Mix, just add sample.
- Compatible with Automated and High-Throughput workflows.
Original Manufacturer
Satisfaction 100% guaranteed, read Our Promise
Innovated in California, Made in the USA
Description
Performance
Technical Specifications
| Compatibility | Compatible with automated and high-throughput workflows. |
|---|---|
| Equipment Required | Real-Time PCR Instruments capable of detecting HEX™ and Quasar® 670/Cy5 fluorophores. |
| Input Quality | Purified RNA free of enzymatic inhibitors. |
| Processing Time | ≤ 1.5 hours from set up to results. |
| Reagents | Complete and ready to use master mixes. |
| Registration Status | FDA-Emergency Use Authorization CE-IVD certified |
| Sample Input Material | Purified RNA samples isolated from upper respiratory and lower respiratory systems. |
Resources
Documents
FAQ
This test has been authorized by the U.S. FDA under an EUA and CE-IVD certified for use by authorized laboratories. In the U.S. this test is only authorized for the duration of that circumstances exist justifying the authorization of emergency use of in vitro diagnostic test for detection and/or diagnosis of COVID-19 under Section 564(b)(I) of the Act, 21 U.S.C. § 360bbb-3(b)(I), unless the authorization is terminated or revoked sooner.
This test has been authorized only for the detection of nucleic acid from SARS-CoV-2, not for any other virus or pathogen.
Please refer to the Interpretation of Results, the Appendix, and the Troubleshooting Guide sections in our Quick SARS-CoV-2 rRT-PCR Kit protocol. If you still have problems with the result interpretation please contact our Technical Support team at (949) 679-1190 ext. 3 or e-mail us at tech@zymoresearch.com.
Sometimes aberrant qPCR signal are observed. Please refer to the Interpretation of Results, the Appendix, and the Troubleshooting Guide sections in our Quick SARS-CoV-2 rRT-PCR Kit protocol. If you still have problems with the result interpretation please contact our Technical Support team at (949) 679-1190 ext. 3 or e-mail us at tech@zymoresearch.com.
This may indicate incorrect plate set-up or a contamination in the No-Template Control or in the CV Mix 1 and/or 2. We recommend to use a new aliquot of reagents.
This may indicate incorrect plate set-up or the compromise of Quick SARS-CoV-2 reagents. We recommend to use a new aliquot of reagents.
If the host target didn’t amplify or shows CT values above 40, your RNA extraction and/or RT-PCR reaction may have been incorrectly performed. We suggest repeating RNA extraction and the RT-PCR reaction.
Late signal for SARS-CoV-2 viral targets may be indicative of a low viral load in your sample. We suggest repeating the RT-PCR to confirm results.
Viral and human targets are amplified in two separate reactions in order to prevent potential competition between PCR. Detecting viral and human targets in two separate reaction minimizes false negative results even in samples with extremely low viral titer.
With a limit of detection(LoD) as low as 15 GEC/reaction the Quick SARS-CoV-2 rRT-PCR Kit outperforms most of the SARS-CoV-2 molecular tests present on the market.
In addition, the detection of a host target (human Rnase P) allow the identification of insufficient samples, which other tests may classify as negative; this further reduce the chances of the false negative results.
Need help? Contact Us