Behind every Nobel Prize-winning discovery lies a story of innovation, persistence, and a set of right tools. Nobel Prize winners exemplify the curiosity and dedication that drive scientific breakthroughs, uncovering new fields of research. While their pioneering work shines brightly, the research methods and technologies they rely on often work quietly in the background, fueling discoveries that change the world.
In this article, we will spotlight some of these remarkable Nobel Prize winners and their groundbreaking contributions to science. Along the way, we will explore some of the cutting-edge RNA techniques that supported their research, which are accessible to laboratories everywhere, helping to shape the future of scientific discoveries.
Victor Ambros and Gary Ruvkun
The Nobel Prize in Physiology or Medicine 2024
“for the discovery of microRNA and its role in post-transcriptional gene regulation”
In 1993, Victor Ambros and Gary Ruvkun made a groundbreaking discovery that revolutionized our understanding of gene regulation. While every cell in the body contains the same genetic information, different cell types, such as muscle or nerve cells, exhibit distinct characteristics due to selective gene regulation. MicroRNAs, as discovered by Ambros and Ruvkun, are a new class of tiny RNA molecules that play a crucial role in gene regulation, ensuring that each cell activates only the relevant genes for its specific function.
Their research, particularly in the model organism C. elegans, revealed that these small non-coding RNA molecules regulate vast networks of gene expression at the post-transcriptional level, controlling mRNA stability and protein production. Ambros and Ruvkun’s work revealed how intricate the regulation of genetic information can be, shedding light on the complex processes of microRNA gene regulation that allow multicellular organisms to develop and function in a highly coordinated manner.
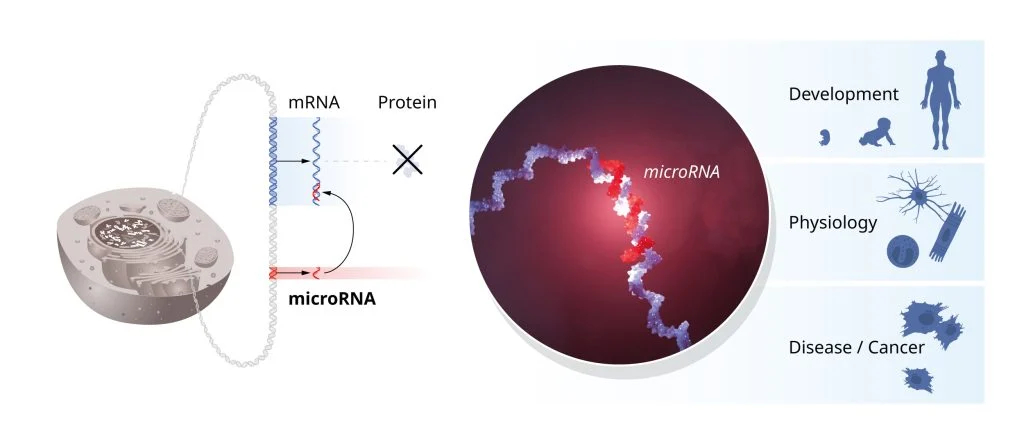
Figure 1. The discovery of microRNAs revealed a novel layer of gene regulation and set new directions for research in developmental biology, physiology, and the understanding of disease mechanisms.
Illustration: © The Nobel Committee for Physiology or Medicine. Ill. Mattias Karlén
Press release: The Nobel Prize in Physiology or Medicine 2024 - NobelPrize.org
Used by Nobel Laureates — Provided by Zymo Research

Katalin Karikó and Drew Weissman
The Nobel Prize in Physiology or Medicine 2023
“for their discoveries concerning nucleoside base modifications that enabled the development of effective mRNA vaccines against COVID-19”
Katalin Karikó and Drew Weissman revolutionized vaccine science through their pivotal discoveries regarding mRNA, which have played a crucial role in developing vaccines that combat infectious diseases. In 2005, the two scientists discovered that specific modifications to RNA’s building blocks could prevent unwanted immune reactions while enhancing protein production. This breakthrough laid the foundation for the highly effective mRNA vaccines used during the COVID-19 pandemic, starting in 2020. Their work showed how mRNA could be modified and delivered into the body to trigger a protective immune response without using live or attenuated viruses, a significant advantage over traditional vaccines.
The impact of their mRNA vaccine breakthrough became particularly evident when companies like Pfizer/BioNTech and Moderna used their technology to develop COVID-19 mRNA vaccines that were rapidly deployed worldwide. These mRNA vaccines successfully generated a strong immune response, including producing high levels of antibodies, which have been crucial in reducing severe illness and death from COVID-19. Karikó and Weissman’s pioneering works have transformed our understanding of how mRNA can interact with the immune system, which have opened new avenues for vaccine development and other therapeutic applications.
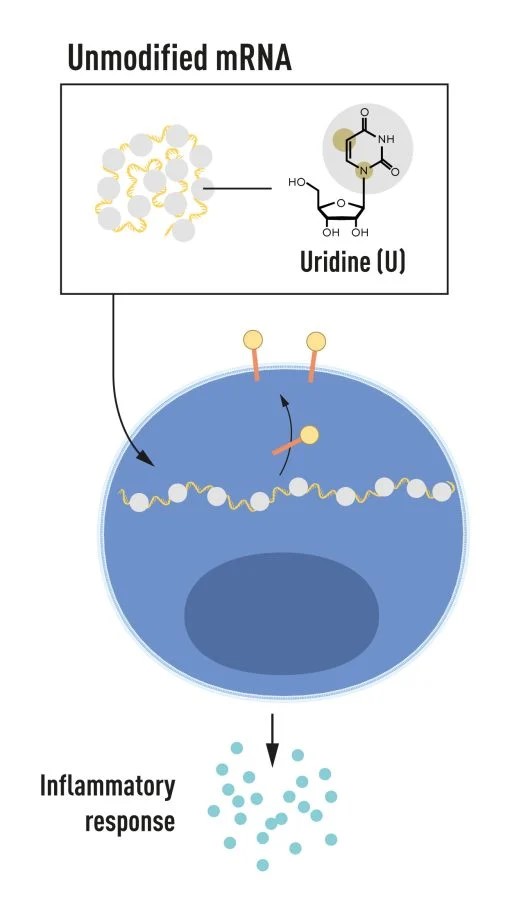
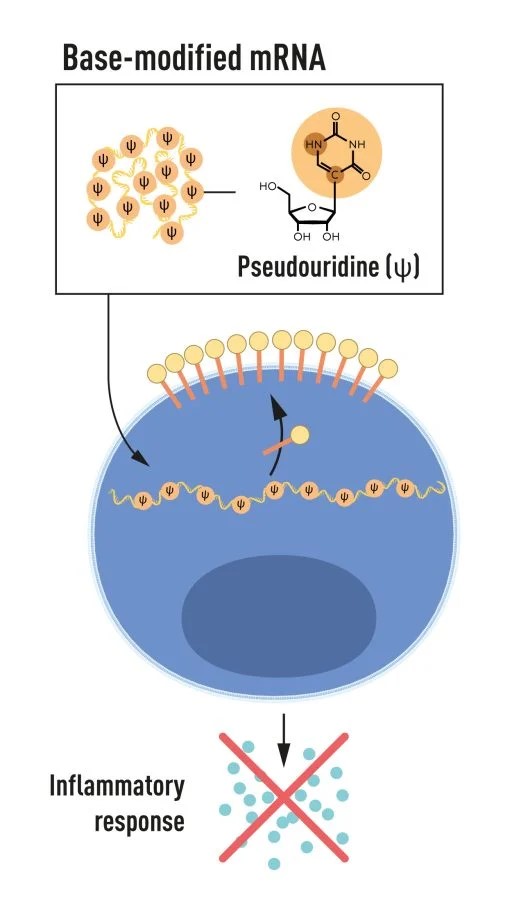
Figure 2. Katalin Karikó and Drew Weissman discovered that base-modified mRNAs can be delivered to cells to alter protein production and prevent inflammatory responses.
Illustration: © The Nobel Committee for Physiology or Medicine. Ill. Mattias Karlén
Press release: The Nobel Prize in Physiology or Medicine 2023 - NobelPrize.org
Used by Nobel Laureates — Provided by Zymo Research

Svante Pääbo
The Nobel Prize in Physiology or Medicine 2022
“for his discoveries concerning the genomes of extinct hominins and human evolution”
Svante Pääbo’s groundbreaking work has significantly advanced our understanding of human evolution. In 2010, he achieved the remarkable feat of Neanderthal genome sequencing, revealing the genetic relationship between Neanderthals and modern humans. His research also uncovered a previously unknown hominin species, the Denisovans, based on genetic material from a mere finger bone. Most notably, Pääbo’s studies demonstrated that interbreeding occurred between Homo sapiens and these extinct hominins, resulting in gene flow that continues to affect present-day humans, such as influencing the physiology of immune responses.
Awarded the 2022 Nobel Prize in Physiology or Medicine, Svante Pääbo have transformed the field of paleogenomics on how we explore human origins and evolutionary biology. His research has highlighted the intricate genetic connections between ancient and modern humans, providing insights into what makes us uniquely human.
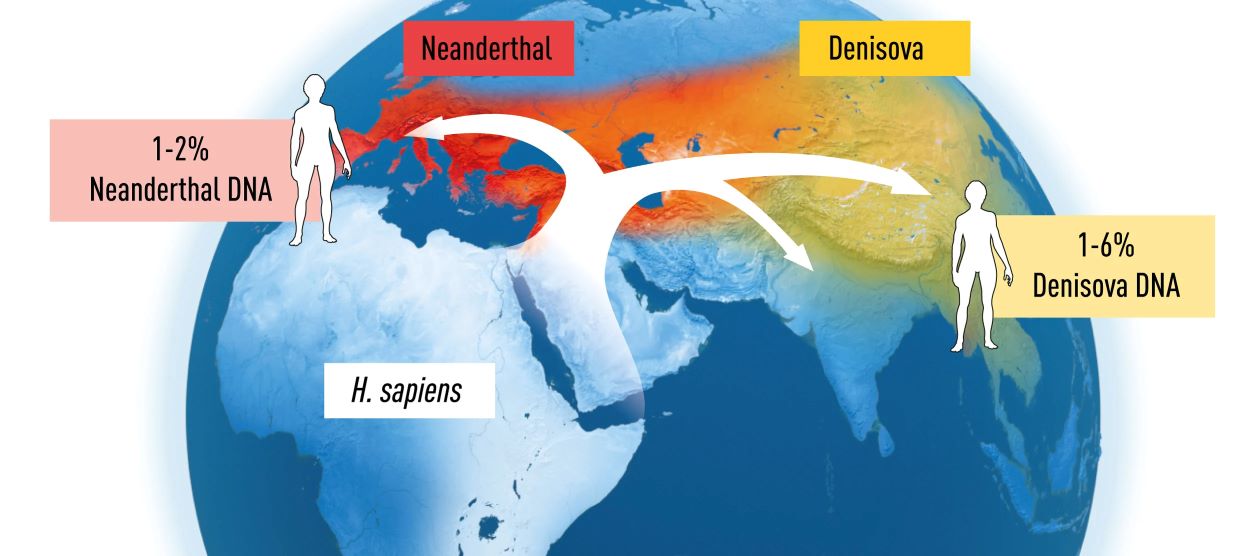
Figure 3. Svante Pääbo’s research revealed the gene flow between Homo sapiens and extinct hominins. Modern-day individuals of European or Asian descent can carry up to 2% of Neanderthal DNA, while individuals in Melanesia and other parts of South East Asia can carry up to 6% of Denisova DNA.
Illustration: © The Nobel Committee for Physiology or Medicine. Illustrator: Mattias Karlén
Press release: The Nobel Prize in Physiology or Medicine 2022 - NobelPrize.org
Used by Nobel Laureates — Provided by Zymo Research

David Julius and Ardem Patapoutian
The Nobel Prize in Physiology or Medicine 2021
“for their discoveries of receptors for temperature and touch”
David Julius and Ardem Patapoutian were jointly awarded the 2021 Nobel Prize in Physiology or Medicine for their pioneering discoveries of receptors that allow humans to sense temperature and touch. Julius’s research in the late 1990s used a cDNA library from sensory neurons to identify a gene that grants sensitivity to capsaicin, the active compound in chili peppers. This led to the discovery of a novel ion channel, the TRPV1 receptor, which senses heat. In the early 2000s, Patapoutian used a functional screen of genes in a pressure-sensitive cell line to identify the PIEZO1 and PIEZO2 receptors, responsible for sensing pressure and touch. These discoveries have revolutionized our understanding of how our nervous system perceives temperature and mechanical forces, unveiling critical mechanisms that underlie touch, temperature sensation, and pain. Their work has provided key insights into the molecular basis of these sensory processes, with wide-ranging implications for medical research, especially in areas like chronic pain and other sensory disorders.
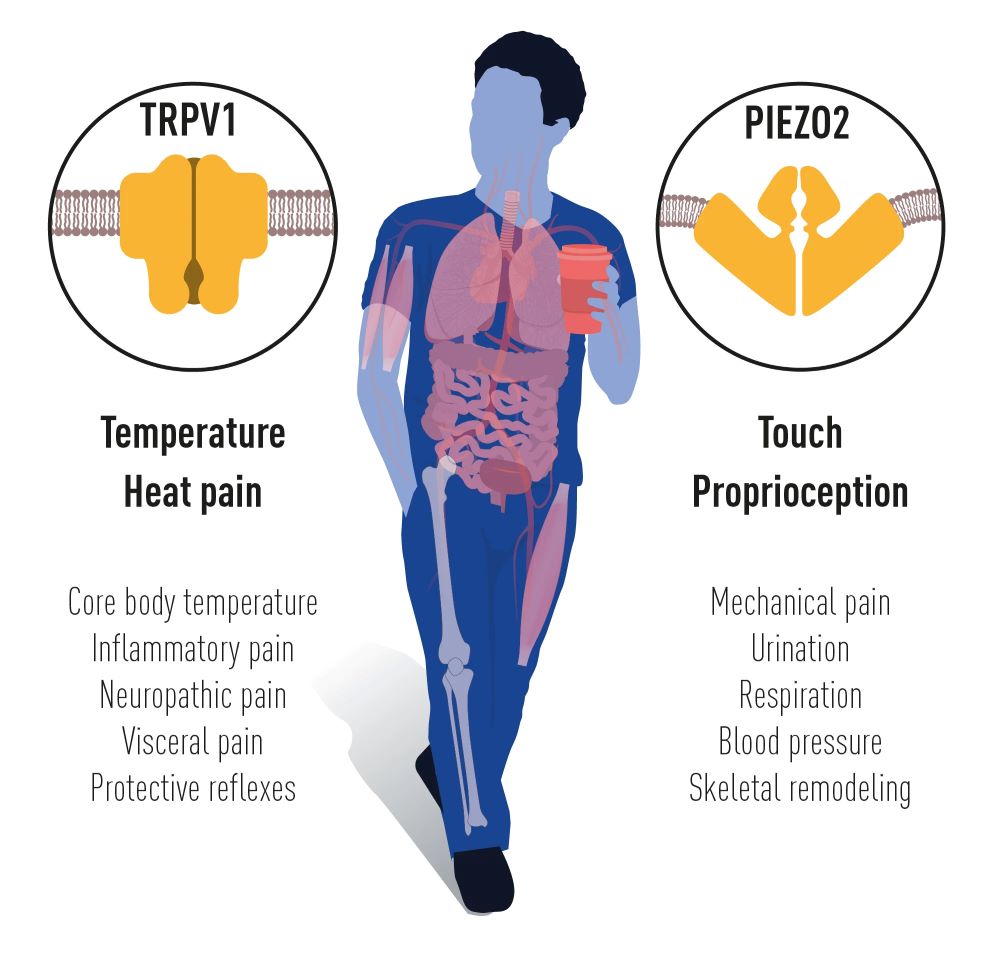
Figure 4. David Julius and Ardem Patapoutian have identified ion channels that are crucial to our nervous system to initiate signals for heat and touch.
Illustration: © The Nobel Committee for Physiology or Medicine. Illustrator: Mattias Karlén
Press release: The Nobel Prize in Physiology or Medicine 2021 - NobelPrize.org
Used by Nobel Laureates — Provided by Zymo Research

Emmanuelle Charpentier and Jennifer A. Doudna
The Nobel Prize in Chemistry 2020
“for the development of a method for genome editing”
In 2012, Emmanuelle Charpentier and Jennifer Doudna revolutionized genetic research by developing a method for precise genome editing. They drew inspiration from the immune system of bacteria, which use molecular "scissors" to cut viral DNA and protect themselves from infections. By isolating and simplifying these CRISPR/Cas9 genetic scissors, they created a powerful tool that allows researchers to edit DNA with exceptional accuracy. This technology has since opened new doors for scientific breakthroughs, enabling the editing of DNA in animals, plants, and microorganisms. CRISPR/Cas9 is now being used to develop more resilient crops, explore potential cancer therapies, and fight against genetic disorders. In recognition of their groundbreaking work, Charpentier and Doudna were awarded the 2020 Nobel Prize in Chemistry. CRISPR gene editing has not only reshaped genetic research but also holds immense promise for advancing medicine and solving critical challenges in health and agriculture.
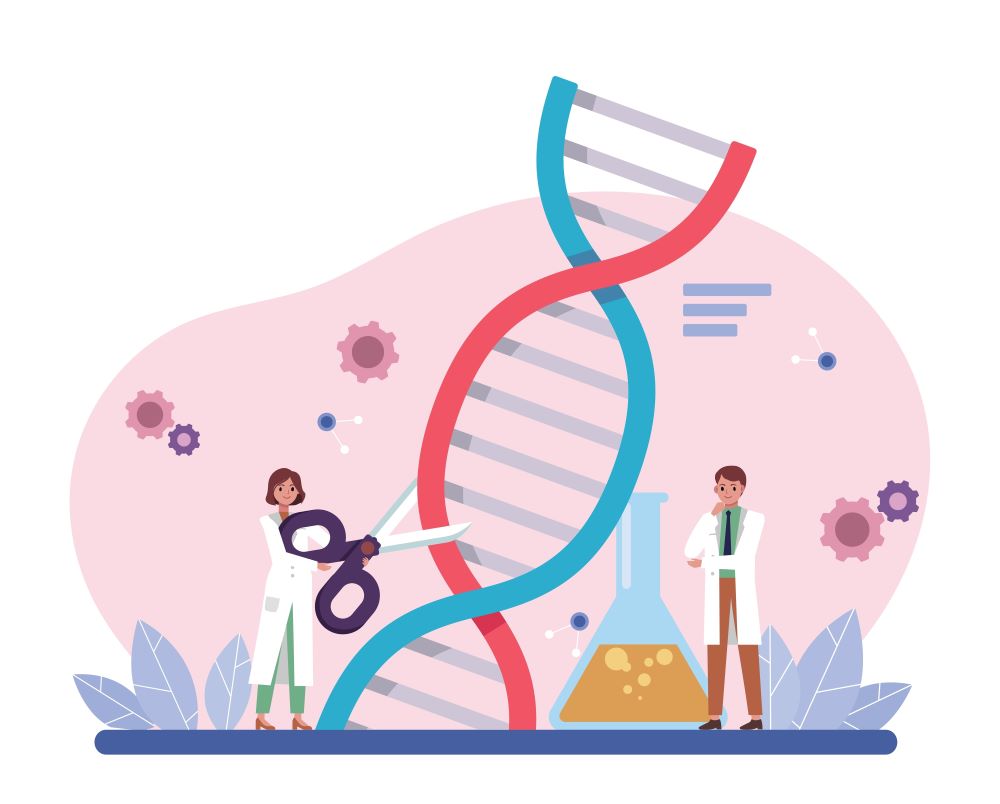
Figure 5. Emmanuelle Charpentier and Jennifer A. Doudna discovered a novel tool for genome editing, enabling scientists to make precise changes to DNA sequences in a wide range of cells and organisms.
Used by Nobel Laureates — Provided by Zymo Research

Harvey J. Alter, Michael Houghton and Charles M. Rice
The Nobel Prize in Physiology or Medicine 2020
“for the discovery of Hepatitis C virus”
Harvey J. Alter, Michael Houghton, and Charles M. Rice were awarded the 2020 Nobel Prize in Physiology or Medicine for their groundbreaking discoveries surrounding the Hepatitis C virus. In the 1970s, Alter's studies revealed a previously unknown contagion, which he demonstrated was a virus, transmitted Hepatitis via blood transfusions. This finding laid the foundation for identifying the Hepatitis C virus. Houghton isolated the virus’s genome in 1989, revealing it as an RNA virus from the Flavivirus family. Rice's work in 1997 proved the virus alone caused Hepatitis C by showing a crucial viral genome region responsible for the disease. These discoveries have dramatically improved public health by enabling the development of screening methods that reduced hepatitis transmission via blood transfusions, as well as effective antiviral treatments that have saved millions of lives worldwide.
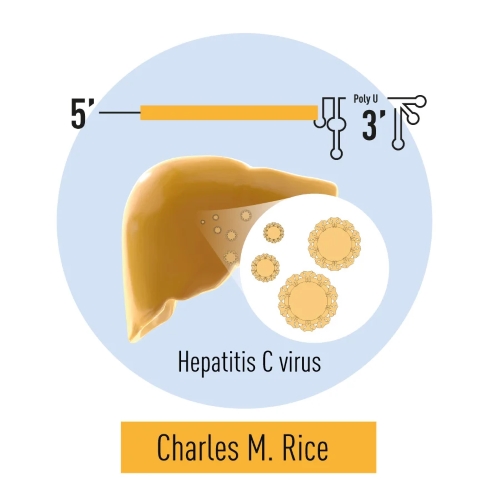
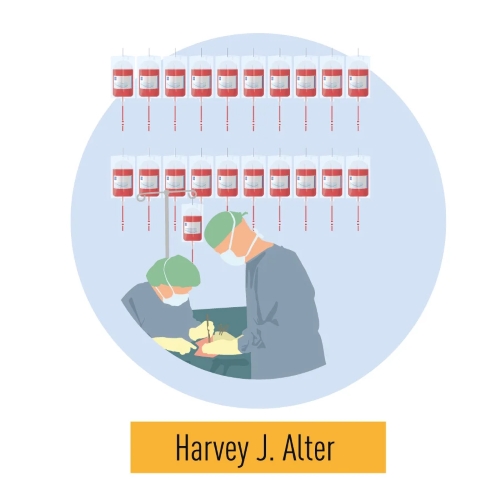
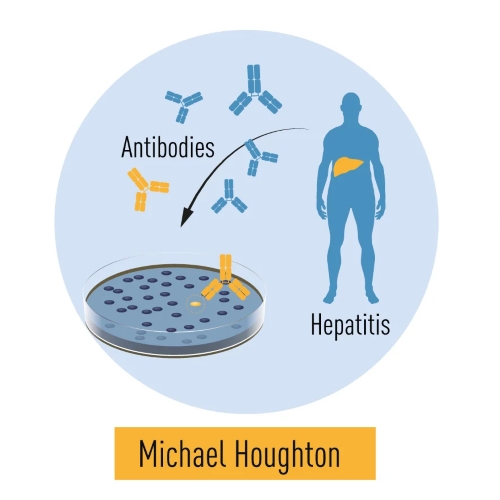
Figure 6. Harvey J. Alter’s studies revealed that a distinct virus was responsible for post-transfusion hepatitis. Michael Houghton utilized antibodies from hepatitis patients to identify the virus, naming it Hepatitis C. Charles M. Rice’s research identified a conserved region of the virus’s RNA genome and demonstrated that the virus alone is capable of causing hepatitis.
Illustration: © The Nobel Committee for Physiology or Medicine. Illustrator: Mattias Karlén
The Nobel Prize in Physiology or Medicine 2020 - Press release - NobelPrize.org
Used by Nobel Laureates — Provided by Zymo Research

The Best RNA Solutions for Your Research
Today, these Nobel Prize winners, along with many others in the field, continue to push the boundaries of science using the most advanced tools available. We are proud to say that Zymo Research’s RNA research tools are part of their ongoing effort, providing the precision and reliability necessary to support groundbreaking discoveries.
Our most cited RNA extractions kits are the Direct-zol RNA Kits and the Quick-RNA Kits. They are designed to purify high-quality, highly concentrated, DNA-free RNA for sensitive downstream analysis including reverse transcription polymerase chain reaction (RT-PCR) and Next Generation Sequencing (NGS). Explore our best RNA purification tools below.
