Successfully Added to Cart
Customers also bought...
-
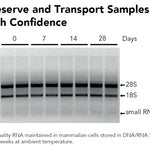 DNA/RNA Shield (50 ml)Cat#: R1100-50DNA/RNA Shield reagent is a DNA and RNA stabilization solution for nucleic acids in any biological sample. This DNA and RNA stabilization solution preserves the...
DNA/RNA Shield (50 ml)Cat#: R1100-50DNA/RNA Shield reagent is a DNA and RNA stabilization solution for nucleic acids in any biological sample. This DNA and RNA stabilization solution preserves the... -
 DNA/RNA Shield SafeCollect Swab Collection Kit (1 ml fill) (1 collection kit)Cat#: R1160The DNA/RNA Shield SafeCollect Swab Collection Kit is a user-friendly collection kit for stabilizing the nucleic acid content of samples collected with a swab. DNA/RNA...
DNA/RNA Shield SafeCollect Swab Collection Kit (1 ml fill) (1 collection kit)Cat#: R1160The DNA/RNA Shield SafeCollect Swab Collection Kit is a user-friendly collection kit for stabilizing the nucleic acid content of samples collected with a swab. DNA/RNA...
Format
Direct-zol RNA Miniprep Plus Kits
Highlights
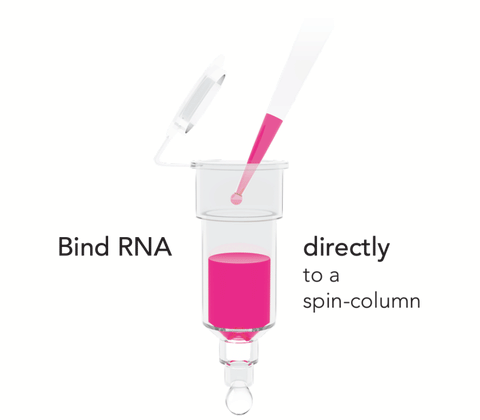
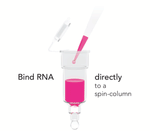
- Easy Handling: Bypass chloroform, phase separation and precipitation steps.
- NGS-Ready: Ultra-pure RNA without phenol carryover. No DNA contamination (DNase I included).
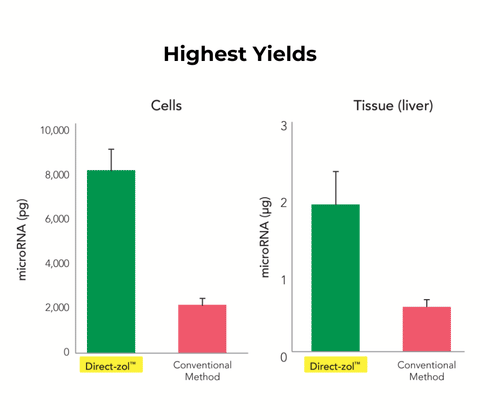
- Non-Biased: Complete RNA recovery without miRNA loss.
Original Manufacturer
Satisfaction 100% guaranteed, read Our Promise
Innovated in California, Made in the USA
Format
Direct-zol RNA Miniprep Plus Kits
Highlights
- Easy Handling: Bypass chloroform, phase separation and precipitation steps.
- NGS-Ready: Ultra-pure RNA without phenol carryover. No DNA contamination (DNase I included).
- Non-Biased: Complete RNA recovery without miRNA loss.
-
MicroprepSample Available
-
Best Seller
MiniprepSample Available
-
Miniprep Plus
-
96-Well
-
MagBead
-
Customize or Order in Bulk
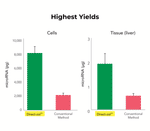
*For best performance with tissue samples, please select Miniprep Plus or MagBeads
Original Manufacturer
Satisfaction 100% guaranteed, read Our Promise
Innovated in California, Made in the USA
| Cat # | Name | Size | |
|---|---|---|---|
| E1010 | DNase I Set | 250 U | |
| R1003-3-12 | RNA Wash Buffer | 12 ml | |
| R1003-3-48 | RNA Wash Buffer | 48 ml | |
| R2072 | Direct-zol RNA Miniprep Plus | 200 preps | |
| R2070 | Direct-zol RNA Miniprep Plus | 50 preps | |
| C1006-50-G | Zymo-Spin IIICG Columns | 50 Pack | |
| C1001-50 | Collection Tubes | 50 Pack | |
| R2050-2-40 | Direct-zol RNA PreWash (Concentrate) | 40 ml | |
| R2050-2-160 | Direct-zol RNA PreWash (Concentrate) | 160 ml | |
| W1001-10 | DNase/RNase-Free Water | 10 ml | |
| W1001-30 | DNase/RNase-Free Water | 30 ml | |
| W1001-1 | DNase/RNase-Free Water | 1 ml | |
| E1010-1-4 | DNA Digestion Buffer | 4 mL | |
| R2050-1-200 | TRI Reagent | 200 ml | |
| R2050-1-50 | TRI Reagent | 50 ml | |
| E1010-1-16 | DNA Digestion Buffer | 16 mL | |
Description
Performance
Technical Specifications
| Compatibility | TRIzol®, RNAzol®, QIAzol®, TriPure™, TriSure™ and all other acid-guanidinium-phenol based solutions can be used in place of TRI Reagent®. |
|---|---|
| Equipment | Microcentrifuge, vortex |
| Sample Inactivation | TRI Reagent® (provided with R2071, R2073) inhibits RNase activity and inactivates viruses and other infectious agents. |
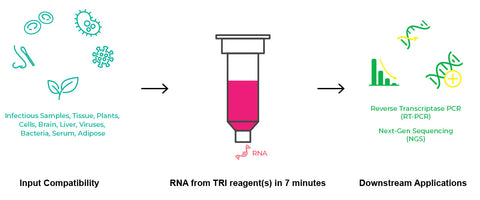
| Sample Source | Any sample stored and preserved in TRI Reagent®, TRIzol® or similar (animal cells, tissue, bacteria, yeast, fecal, biological fluids, and in vitro processed RNA (e.g., transcription products, DNase-treated or labeled RNA)). |
| Size Range | Total RNA ≥ 17 nt |
| Yield | 100 µg RNA (binding capacity), ≥ 50 µl (elution volume) |
Resources
Documents
FAQ
Yes, the Direct-zol MicroPrep (#R2060) is designed and capable of purifying RNA down to single cell inputs (picogram amounts). A sensitive quantification method is needed (e.g. Qubit, qPCR, etc.)
All kit components are available for purchase separately.
Lyophilized DNase I is stable at room temperature. Once resuspended, store frozen aliquots. Minimize freeze thaw cycles as much as possible. Freeze thaw will lower DNase activity.
If the downstream application requires DNA-free RNA, we recommend performing the DNase I treatment.
Yes, bring samples homogenized and stored in DNA/RNA Shield to room temperature (20-30ºC). Add an equal volume (1:1) of TRI Reagent®(s) and mix well. Proceed with RNA Purification.
Yes, proteins can be acetone precipitated from the column flowthrough. Please see the Protein Purification appendix in the protocol.
Yes, samples in TRIzol/TRI Reagent or similar are stable overnight at room temperature and can be stored frozen (-80C). Be sure to lyse and homogenize the sample well prior to freezing. Bring the sample to room temperature prior to RNA Purification.
Direct-zol DNA/RNA (R2080) kits can isolate DNA from TRI Reagent®(s).
Yes, the RNA is high quality (A260/A280 >1.8, A260/A230 >1.8) and suitable for any downstream application, including NGS, RT-PCR, hybridization, etc.
The Direct-zol kits are compatible with TRI Reagent, Qiazol, RNAzol, TriPure, TriSure, etc., and any other acid-guanidinium phenol-based reagents.
Direct-zol is for samples stored/collected into TRI Reagent®(s). Quick-RNA is for all other samples.
Both kits function the same, the only difference is the RNA binding capacity of the column provided with the kit.
Yes, use 80% ethanol as a substitute. RNA Wash Buffer is also sold separately.
Yes, the Direct-zol RNA kits are compatible with CTAB-based RNA extraction methods for polysaccharide-rich and/or phenolics-rich samples (e.g., Pinus, Geranium plants). Please find detailed protocol here.
Purity, RIN and/or any type of contamination can result from initial sample preparation (i.e., inefficient lysis of the sample). To improve, increase the volume of the lysis reagent (e.g., TRI Reagent®(s) or RNA Lysis Buffer).
- To ensure complete lysis, increase the volume of the lysis reagent (i.e., increase or titrate the volume of TRI Reagent/TRIzol or RNA Lysis Buffer). Lysate should be clear (not opaque or viscous). Pellet debris by centrifugation (if needed) and process the cleared supernatant.
- Prior to adding TRI Reagent/TRIzol or RNA Lysis Buffer, perform enzymatic treatment (Proteinase K; #D3001-2-20) and/or mechanical homogenization (bead beating with ZR BashingBead Lysis Tubes; #S6003) in DNA/RNA Shield (#R1100).
Reviews
"It took hardly any time, the protocol was so easy, and my RNA quality was SO much better. Honestly, this kit revolutionized my life at the bench."
A. Newhart
The Wistar Institute
"Simple protocol and yielded good quality of RNA. Only one kit working for all type of tissue, cell and especially biological fluids."
Mohan K.
University of Illinois, Chicago
"Previously I used a protocol that took 3 hours, now I can have my RNA in 20 minutes. What is not to like about that? Just one column and two buffers, I love it."
Arjan V.
Indiana University
Citations
Registered trademarks: TRI Reagent®, TRIzol® and RNAzol® (Molecular Research Center, Inc.), QIAzol® (Qiagen GmbH), TriPure™ (Roche, Inc.), TriSure™ (Bioline Ltd.)
Need help? Contact Us